What is plastic? An introduction to Polymer Science
EDUCATIONPOLYMER

Plastic is one of these Buzzwords that you hear everywhere. They are part of your daily lives, in your clothes, your car and even your food. But what is plastic? Behind this umbrella term hides a wide variety of materials with very different properties that are part of the same family: polymers. In this article, we'll go back to the fundamental principles of polymer science and break them down so you can finally understand what is plastic.
What are polymers?
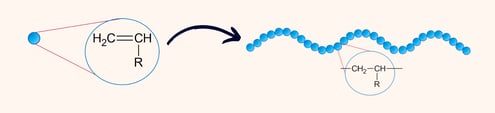
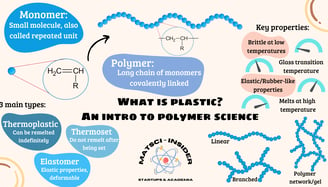
Plastics are polymers, which are long chains of repeating molecular units called monomers. Think of polymers as a necklace and monomers as the beads. These monomers are linked by covalent bonds to form the backbone of the material. Polymers can occur naturally, like DNA and proteins, where building blocks are nucleic and amino acids. Plastics, on the other hand, are synthetic polymers specifically engineered for particular uses. Their building blocks are also organic, but do not necessarily occur in nature.
The length and arrangement of these chains play a crucial role in determining the properties of the plastic.
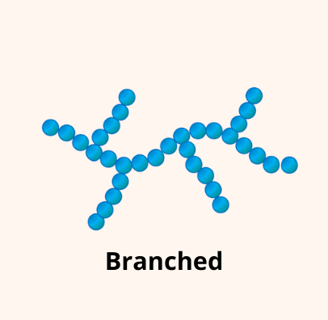
For example, longer polymer chains usually make the material stronger and more durable. Polymers can have different structures—linear, branched, or cross-linked—each leading to unique properties. The degree of branching can be controlled during the synthesis to obtain these properties. The same goes for cross-links, chemical bonds that join two different strands of polymer together, creating a strongly bonded network structure.
Other weaker forces also play a role in the formation of a coherent structure. Van der Waals forces, caused by the interactions of electrons, can play this role by forcing molecules to stick together. These bonds often break upon heating, leaving polymer chains free to move independently. Hence, polymer primarily formed without cross-links are able to melt and are called thermoplastics.
Cross-links require much more energy to break. Polymers formed with a high amount of cross-linked chains are called thermosets and are unable to melt. When thermosets are heated at a high enough temperature, the chemical bonds break and the whole polymer decomposes.
Polymers with a small degree of cross-linking are called elastomers. Few cross-links allow the polymer to be less rigid while preventing the chains from simply sliding past each other when heated. This gives the polymer its "elastic" properties